An introduction to the em-model
3 Light and matter
How a classical oscillations can explain observed
electromagnetic radiation
3.5.04 Erling Skaar
Can quantum properties be explained by the
EM-model?
In the previous two documents we have described matter according
to the EM-model. But these documents is probably not enough to persuade those that believe that
the QM-model (Quantum Mechanics model) is the only model
that can describe nature, that there may be an alternative model that also
may describe nature. A reason is that we have not mentioned
the main problem in classical physics that led to a general acceptance of the
QM-model about 100 years ago. Matter exchange energy in form of light and other
electromagnetic radiation. A main view is that because classical physical
principle could not explain different properties connected to this exchange
of energy, scientist had to accept the QM-model.
|

According
to the QM-model, light is a sort of particle (photons) with energy
exact like the difference between two energy levels of an electron.
|
|

According
to the EM-model, light is a sort of wave-trains which originates
in oscillating electrons which have different oscillating or resonant frequency.
|
A main difference between the
QM-model and the EM-model(ElectroMagnetic mode) is that the EM-model is based on physical explanations
where continuous values is what we expect in basic explanations. The QM-model
on the other hand is based on mathematical equations. A main assumption in the
QM-model
is that everything in nature, both matter and electromagnetic waves, are quantified
in small exact values called quantum.
A main purpose in this document is to show
that the EM-model based on classical electromagnetism can explain the experiments
that bothered scientists about 100 years ago and those that bother modern scientists
today. Because the QM-model have a total dominance in the science communities
today,
other models have experienced straitened circumstances. The EM-model in its
present state is rather new and much work is ahead of us. Therefore it should
be accepted that this short introduction can not cover everything that
is relevant, but some main evidences for the EM-model and some main problems
for the QM-model will be mentioned.
Two main substances in nature: Matter and electromagnetic
radiation
A main aim behind these documents is to help everybody, not only physicists,
to understand nature. Some general cognitive structures (thinking structures)
is therefore valuable. In seems that those that supports the QM-model
do not always estimate efforts to make simple explanation of basic processes in nature. The reason is
probably that it is not possible to make a simple description of the nature based on the QM-model.
As mentioned in the previous two documents, matter consists of two elementary
particles with two sorts of forces in between them according to the EM-model. But if this was all that
exists in nature we could not exist. Energy is another fundamental property
in nature which is mainly exchanged between elements/structures as electromagnetic radiation. We need both
light and heat to live and both of these are called electromagnetic radiation. In general we can say that light is energy that comes from elements and ends
up in elements. We says that elements emit light, and absorb light and when
light is on its way from where it is emitted to where it is absorbed we often
call it electromagnetic waves. That is because it has been shown that light
is of the same nature as other electromagnetic waves like radio waves, microwaves
and infrared radiation or heat radiation.
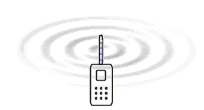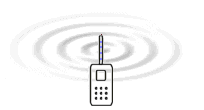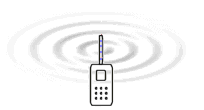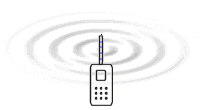
Electromagnetic
waves is created because electrons are forced to oscillate up and
down in the antenna. It is the electric field which surrounds all
charges that is the medium that is waving and transports the energy
according to the EM-model.
|
|
em-radiation
|
Frequency
|
|
Light
|
around 10-15
|
|
Infrared(heat)
|
10-12-10-14
|
|
Microwave
|
10-9-10-12
|
|
Radio wave
|
less than 10-9
|
The only difference between these radiations is the frequency. The table to the
left gives a overview of the frequencies that are involved in different radiation
or waves. The animation to the right illustrates how energy in form of waves leaves
the antenna of a mobile telephone. The nature of the energy that radiates from
the antenna have been a main question all the time since the radio was developed.
According to modern physics (the QM-model), all radiation from light to
radio waves is quantified into something called photons which is a sort of energy
packets or particles.
Although this is the accepted view among orthodox scientists, we experiences
in practical situations that more and more science books describes this
sort of radiation as waves.
Problem to understand the QM-model
The quantum explanation of electromagnetic
wave is hard to understand partly because of some internal
paradoxes in the explanations. The best known paradox, which the believers of
the QM-model may say are not a paradox, can be addressed with a question: How
can light (or other electromagnetic radiation) be both a wave and a particle
(photon) at the same time? Those that have studied real waves or real particles
knows that a wave have to include at least a wavelength in the direction of
radiation, but it have also an extension normal to its velocity (se the animation
above). A particle on the other hand, have a rather clear surface or border
and therefore it makes cense to measure how big a particle is. The wavelength
of the radiation from a mobile can be measured in cm, and the wavelength
of for example the radio band LW can be measured in km. One problem connected
to the wave-particle-paradox that faces those that believe that all electromagnetic
radiation is quantified in photons, is to figure out how big those particles
are that are called photons. Because of that sort of problems, it is common
practice to not mention the QM-model while working with electromagnetic radiation.
The EM-model have shown that it works with low frequencies - why not use
it on high frequencies?
A possible question that may be asked is the why stick to the QM-model while
it seems to create major problems to those that tries to understand what really
goes on in a radio or mobile telephone. It is a fact that those that work with electromagnetic
communication uses the classical electromagnetic explanation in their writings
and work. It means that they explains what happens in for example the mobile telephone above
as oscillation of electrons in the antenna which then makes electromagnetic
waves in the surroundings which is often called the ether. These
waves will then reach another antenna and makes some electrons
oscillate. They do not describe the process as a matter of electrons jumping from one state to another
as would be a logical conclusion based on the QM-model.
A conclusion so long is that people uses classical oscillation models
when explaining electromagnetic waves with low frequency, but the orthodox scientists
sticks to the QM-model when they explains light and electromagnetic radiation
with higher frequency. It has also been a strong and effective
resistance against different suggestions that also light interactions with matter
may be explained by using oscillating electron is stead of jumping electrons.
If people with high positions in scientific communities wants to withhold
the common explanation of light, it seems that they may do it by just refuse to listen
to the evidence against the QM-model. That tactic will work as long as other
people
thinks that scientific truth is what the experts says. Her we will just encourage
the reader to make some own thinking instead of just accepting what the scientific authorities
says. This is not about forbidding the QM-model but let the EM model get a fair
chance to show its value.
 The EM-model is based on classical electromagnetism and therefore there are
no problems with explaining electromagnetic radiations with low frequencies.
The EM-model is not a new explanation.
What we here may call new, is the idea that all light phenomena
can be explained with an electromagnetic model. At first look it may
seem a little "single tracked" to just accept one explanation when
those with the other view (the QM-model), seems to accepts electromagnetic
explanations in many cases. But
this is about understanding the fundaments in nature, and therefore it is important
to avoid everything that may create confusion. An analogy: Someone may argue that
those that accepts two wives is more kind than those that just accept one. If the purpose is to get a good marriage, many will agree that the more different
thinking which we include, the more probable is conflict and confusion.
The EM-model is based on classical electromagnetism and therefore there are
no problems with explaining electromagnetic radiations with low frequencies.
The EM-model is not a new explanation.
What we here may call new, is the idea that all light phenomena
can be explained with an electromagnetic model. At first look it may
seem a little "single tracked" to just accept one explanation when
those with the other view (the QM-model), seems to accepts electromagnetic
explanations in many cases. But
this is about understanding the fundaments in nature, and therefore it is important
to avoid everything that may create confusion. An analogy: Someone may argue that
those that accepts two wives is more kind than those that just accept one. If the purpose is to get a good marriage, many will agree that the more different
thinking which we include, the more probable is conflict and confusion.
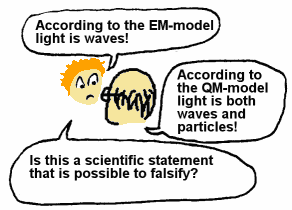
A basic principle in science is also that it should be possible to falsify
scientific statements based on experiments or observations. Some statements
may be so general that it is impossible to describe experiments or observations
which can test the statement, and in that case we should not call the statement
scientific.
Ockham's Razor
is a common name for a principle that in short say that the easiest explanation
is normally the best. A simple explanation is normally better than a more complicated
one as long as we don't know any phenomena that it cannot explain. But if
we discover phenomena that the model can't explain we should first consider a
reversion before we chose a model that can explain almost everything as seems the case
for the QM-model. If a fundamental
assumption in the QM-model says that things can be considered both waves and
particles which are logically opposite explanations, you probably have got a
model that can explain everything. That sort of theories are normally not
very useful in science. If anybody do not agree with this statement, I'm still
searching for descriptions of experiments or observations which may
falsify the QM-model. If they exists I want to hear about them.
The EM-model
Before we comments on how the EM-model explains some fundamental experiments,
we will give a general introduction to some main principle.
1) It is mainly oscillations of electrons that are involved in emission and
absorption of electromagnetic waves. Protons and whole nucleuses are more heavy
(more magnetic energy) and therefore they will oscillate less.
2) Electrons have to be a part of a structure to oscillate. In our daily life
we experience many things that oscillate (pendulum, strings on a guitar etc.)
and a main condition for oscillations is that there are forces that drags
the swinging particle back to a point of equilibrium. In atomic structures
it is electric and magnetic forces with opposite direction that force the electron
back when it swings out of its equilibrium.
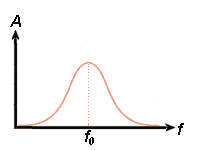 3)
A single electron in a structure may be compared with a pendulum which may be set into oscillations
by for example periodical pushes. The pendulum will have a special resonance
frequency(f0) and if the external influence have that frequency the pendulum will
get the biggest amplitude (A). Frequencies near to resonance frequency may also
result in oscillations and therefore we get a frequency-amplitude-diagram
as shown to the right if a single electron oscillates.
3)
A single electron in a structure may be compared with a pendulum which may be set into oscillations
by for example periodical pushes. The pendulum will have a special resonance
frequency(f0) and if the external influence have that frequency the pendulum will
get the biggest amplitude (A). Frequencies near to resonance frequency may also
result in oscillations and therefore we get a frequency-amplitude-diagram
as shown to the right if a single electron oscillates.
4) Electrons will absorb electromagnetic radiation and start to oscillate
when they are hit by electromagnetic radiation with frequency near to the resonance
frequency. On the other hand, when electrons oscillate, they will emit electromagnetic radiation
with the oscillating frequency. In general an electron will absorb radiation
from one direction and emit it in different directions (circular in 360
degree as shown in the mobile phone animation)
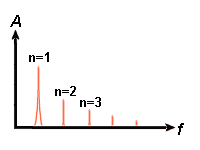 5)
Because all electrons in atomic structures have a spin according to the EM-model,
there is a magnetic force that makes electrons come together into pairs.
That means that when one electron starts to oscillate because of external influence,
it will itself emit radiation with the same frequency and the neighbour-electron
will receive relative much of that radiation and itself start to radiate. We
then gets a situation that is more like a guitar string than a pendulum. To
the right we see three modes which may exist on one a guitar string at the
same time. These are called standing waves or modes. The first mode gives the tone called the key
tone and the others gives
some harmonic tones with higher frequency. The
swinging guitar sting may be considered a wave-guide that transmit waves between
the ends of the string where they are reflected. In this situation we get another
frequency-amplitude-diagram than from the pendulum. A diagram is shown to the
left and the formula that lies behind is:
5)
Because all electrons in atomic structures have a spin according to the EM-model,
there is a magnetic force that makes electrons come together into pairs.
That means that when one electron starts to oscillate because of external influence,
it will itself emit radiation with the same frequency and the neighbour-electron
will receive relative much of that radiation and itself start to radiate. We
then gets a situation that is more like a guitar string than a pendulum. To
the right we see three modes which may exist on one a guitar string at the
same time. These are called standing waves or modes. The first mode gives the tone called the key
tone and the others gives
some harmonic tones with higher frequency. The
swinging guitar sting may be considered a wave-guide that transmit waves between
the ends of the string where they are reflected. In this situation we get another
frequency-amplitude-diagram than from the pendulum. A diagram is shown to the
left and the formula that lies behind is:
where n is a number (n=1,2,3,..), L is the length of the string
and ln is the
wavelength of the wave. If v is the velocity of the wave along the string (v=lf)
the equation may be transformed to:
where fn is the frequency of what we may call frequency
lines or spectral lines. Note that those lines are much
more narrow than the broad "band" around the resonance frequency from
a pendulum. The point here is then that a couple of electrons also will give
what we call spectral lines when they are exposed to energy of one or another
sort.
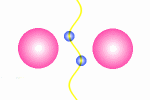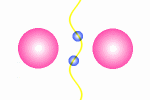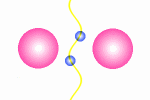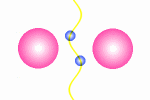
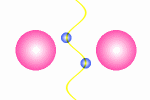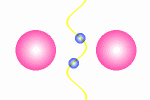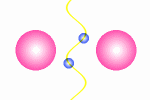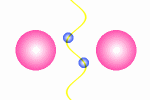
A model of a oscillating electron pair
|

A
couple of electron oscillate with positive interference because
the distance between them is l/2.
|
The principle for a oscillating couple of electrons is shown in the figure
to the right. A light wave is coming in from left and causes the first electron
to oscillate. This oscillation will result in new wave from the first electron
which will propagate in all direction (360░). The part that continue in the
direction toward the next electron will be in phase with the first wave
that started the oscillation. The second electrons will then do the same as
the first, and some of the wave from the second electron will go back toward
the first electron (This wave is not drawn in the animation). In this situation where the first electron receives waves
from both directions, it is essential that they are in phase, otherwise they
will work against each other on the electron and force it to stop oscillating.
The distance between the electrons have to be a l/2,
3l/2 and so fort to avoid that
the waves from the electrons interferes negatively. This means that we from
an electron pair should expect half of the spectral lines than we receive from
a guitar string but the equation is in principle the same:
where n=1,2,3.. and k is a constant if the speed of light
is a constant. This means that a couple of electrons may give spectral
lines according to the same principle as we have in a guitar string if the distance
between them is so big that it is possible to draw one or more wavelength between
them.
Is there enough space for a half wavelength's between electron pairs?
This question address an important problem that is treated more in dept other
places. Here we will just say that the electrons that are the end points for
electromagnetic radiation, is surrounded by an electric field with high density,
and that field will then slow down the speed of light and also make the wavelength
of the electromagnetic waves much shorter. In common
science books we can read that the wavelength of light is about 10-6m
while the size of the atoms is about 10-10m. If we then assume that
an average distance between electrons are 10-11m, it understandable
that most people will conclude that there are not enough space between two electrons
for standing waves which have to be minimum a half wavelength. But this conclusion
is based on the assumption that the speed of light is constant (c=3e8m/s) all
the way between the two electrons. If for example the size of the electrons
are about 10-14m which means a 1/1000 part of the distance between
two electrons, we can then show that the speed of light close to the two electrons
will be so low that there really are enough space for whole wavelengths between
the two electrons.
|

This
figure shows two electrons that exchange waves. The wave fronts
illustrate that the wavelength and also the speed of light will
experience a radical reduction near to the electrons.
|
This is done in some other documents and here we will jus conclude that it is possible to get whole wavelengths
of light between electrons in atomic structures.
So long we have mentioned that electron pair that oscillate will result in
small spectral lines in stead of broad bands of electromagnetic radiation as
we would expect from single electrons. We have also shown that there may be
enough space between electron pairs for a number of wavelengths if we assume
that the speed of light varies as d▓ near to the electrons. An example
of the spectral lines which may be explained by the EM-model is the spectral
lines of Hydrogen which is shown in the figure below:
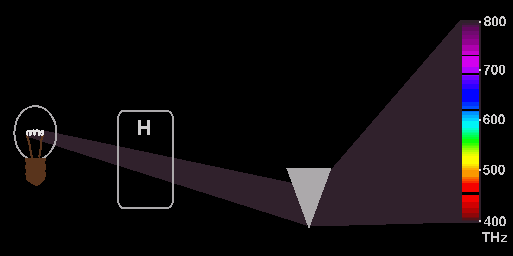 Here
we se that ordinary white light from a light bubble is going through a prism
and is there spread out in different colours. But because the white light also
goes through hydrogen gas, we may experience that some of he light will be absorbed
by the hydrogen gas and this will leave some dimmed or black lines on the spectrum
to the right. This spectrum is then called an absorption spectrum and it is
the explanation behind hose small spectral lines with a special mathematical
relation which also may be explained by the EM-model. Here we will just mention
that they may be explained as caused by standing waves between electrons in
the same way as sound from a guitar string originates from standing waves on
the string. More about his in other documents.
Here
we se that ordinary white light from a light bubble is going through a prism
and is there spread out in different colours. But because the white light also
goes through hydrogen gas, we may experience that some of he light will be absorbed
by the hydrogen gas and this will leave some dimmed or black lines on the spectrum
to the right. This spectrum is then called an absorption spectrum and it is
the explanation behind hose small spectral lines with a special mathematical
relation which also may be explained by the EM-model. Here we will just mention
that they may be explained as caused by standing waves between electrons in
the same way as sound from a guitar string originates from standing waves on
the string. More about his in other documents.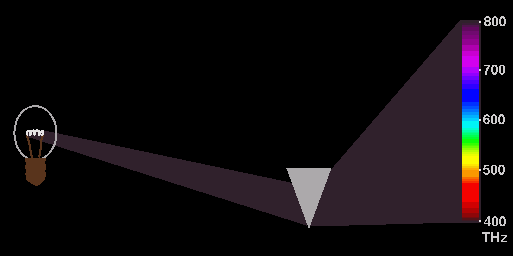
How do the EM-model explains temperature and heat?
In common textbooks and other places, the
concept temperature is connected to the velocity of atoms or molecules. According
to the EM-model, it is electrons (not atoms or molecules) that experience different
forms of vibrations which also sends out heat radiation.
When the EM-model connect the temperature
concept to radiation it means that it makes sense to talk about temperature
also in a evacuated chamber in a normal lab. The temperature outside and inside
will be the about same in this case, but if we connect temperature to the velocity
of the molecules in the chamber we gets some definition problems. It means for
example that it is impossible to talk about temperature if there are no molecules
and if there are for example one cesium atom and one nitrogen molecule, which
of them defines the temperature in the chamber? Common thermometer is based on the principle that things expands while
the temperature rise, an the animation to the right illustrate this principle. The
two red spheres illustrates two positive nucleus in an atomic structure like
a metal for example.
Higher amplitude of the oscillating binding electrons will then result in increased
size of the compound (push the buttons on the figure) and if the temperature
gets too high it means that the bindings may broke down and atoms or molecules
will then escape as gas particles.
 (small copies that makes extra images follow the document)
(small copies that makes extra images follow the document)
Conclusion
Different tests from different fields inside physics have shown that the
EM-model may explain all experiments and observations in a more logical way
than the traditional QM-model can. The work with the EM-model have just started
and there are of course a need for more studies before we can reach a definite
conclusion. A problem which may stop this sort of free debate inside science,
is predisposed people that believe that the QM-model is the only model that
can explain nature. It is a basic idea in science that different scientific
models and explanations should have an equal right to be tested with normal
scientific methods by people which have time and equipment to do this sort of
job, and it is therefore a hope that there exists some scientists somewhere
that are able to give an open minded evaluation of the EM-model.



 The EM-model is based on classical electromagnetism and therefore there are
no problems with explaining electromagnetic radiations with low frequencies.
The EM-model is not a new explanation.
What we here may call new, is the idea that all light phenomena
can be explained with an electromagnetic model. At first look it may
seem a little "single tracked" to just accept one explanation when
those with the other view (the QM-model), seems to accepts electromagnetic
explanations in many cases. But
this is about understanding the fundaments in nature, and therefore it is important
to avoid everything that may create confusion. An analogy: Someone may argue that
those that accepts two wives is more kind than those that just accept one. If the purpose is to get a good marriage, many will agree that the more different
thinking which we include, the more probable is conflict and confusion.
The EM-model is based on classical electromagnetism and therefore there are
no problems with explaining electromagnetic radiations with low frequencies.
The EM-model is not a new explanation.
What we here may call new, is the idea that all light phenomena
can be explained with an electromagnetic model. At first look it may
seem a little "single tracked" to just accept one explanation when
those with the other view (the QM-model), seems to accepts electromagnetic
explanations in many cases. But
this is about understanding the fundaments in nature, and therefore it is important
to avoid everything that may create confusion. An analogy: Someone may argue that
those that accepts two wives is more kind than those that just accept one. If the purpose is to get a good marriage, many will agree that the more different
thinking which we include, the more probable is conflict and confusion.
 3)
A single electron in a structure may be compared with a pendulum which may be set into oscillations
by for example periodical pushes. The pendulum will have a special resonance
frequency(f0) and if the external influence have that frequency the pendulum will
get the biggest amplitude (A). Frequencies near to resonance frequency may also
result in oscillations and therefore we get a frequency-amplitude-diagram
as shown to the right if a single electron oscillates.
3)
A single electron in a structure may be compared with a pendulum which may be set into oscillations
by for example periodical pushes. The pendulum will have a special resonance
frequency(f0) and if the external influence have that frequency the pendulum will
get the biggest amplitude (A). Frequencies near to resonance frequency may also
result in oscillations and therefore we get a frequency-amplitude-diagram
as shown to the right if a single electron oscillates.


 5)
Because all electrons in atomic structures have a spin according to the EM-model,
there is a magnetic force that makes electrons come together into pairs.
That means that when one electron starts to oscillate because of external influence,
it will itself emit radiation with the same frequency and the neighbour-electron
will receive relative much of that radiation and itself start to radiate. We
then gets a situation that is more like a guitar string than a pendulum. To
the right we see three modes which may exist on one a guitar string at the
same time. These are called standing waves or modes. The first mode gives the tone called the key
tone and the others gives
some harmonic tones with higher frequency. The
swinging guitar sting may be considered a wave-guide that transmit waves between
the ends of the string where they are reflected. In this situation we get another
frequency-amplitude-diagram than from the pendulum. A diagram is shown to the
left and the formula that lies behind is:
5)
Because all electrons in atomic structures have a spin according to the EM-model,
there is a magnetic force that makes electrons come together into pairs.
That means that when one electron starts to oscillate because of external influence,
it will itself emit radiation with the same frequency and the neighbour-electron
will receive relative much of that radiation and itself start to radiate. We
then gets a situation that is more like a guitar string than a pendulum. To
the right we see three modes which may exist on one a guitar string at the
same time. These are called standing waves or modes. The first mode gives the tone called the key
tone and the others gives
some harmonic tones with higher frequency. The
swinging guitar sting may be considered a wave-guide that transmit waves between
the ends of the string where they are reflected. In this situation we get another
frequency-amplitude-diagram than from the pendulum. A diagram is shown to the
left and the formula that lies behind is: 
