An introduction to the em-model
2 Structuring elements
22.3.04 Erling Skaar
 In
the previous document we have presented the two fundamental particles (electrons
and protons) and the two fundamental
forces in nature (electric and magnetic force). We also saw that a universe filled
with hydrogen gas is a probably first step toward the universe we know today.
In
the previous document we have presented the two fundamental particles (electrons
and protons) and the two fundamental
forces in nature (electric and magnetic force). We also saw that a universe filled
with hydrogen gas is a probably first step toward the universe we know today.
|
Example from the web:
Elements like hydrogen and helium first formed in the Big Bang.
These grouped
together to form stars where some of the heavier elements are formed. At first they convert hydrogen
atoms to helium - which gives off a lot of energy. Then they start converting the helium to carbon. This process carries on through the periodic table until you
reach iron. Here the process stops because to convert iron to the next in the
series takes more energy than you get out at the other end.
There are other processes in stars called
s-processes (s for slow). Some reactions produce
neutrons which are captured one by one by other nuclei, increasing their weight.
Once a neutron has been captured it may change into a proton.
The s-process can make elements up to bismuth in the periodic table.
Stars can explode
as super nova. In this case there is a flood of neutrons that are absorbed by
existing nuclei several at a time. This is known as the r-process (r for rapid).
This makes elements as heavy as uranium.
http://www.sciencenet.org.uk/database/
chem/elemental/c00099d.html
|
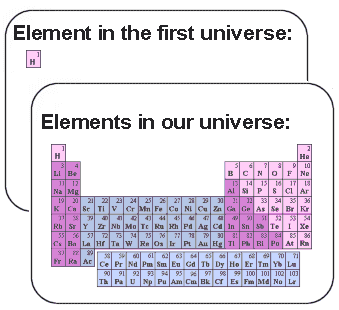
Our universe is filled with about 100 different basic elements
and not only hydrogen, as in the first universe. The main goal for this document
is to look into the problems the creator faced when he had to make other elements
based on the hydrogen gas that filled the first universe.
Common cosmological models
In common cosmological literature
we can read that gas clouds where gravitationally attracted to each other,
formed big stars, which then later exploded (supernova), and the high energy concentration is
these explosions then made the different elements we have today. The frames in this document contains some quotations from some web-site which represent a common view
among scientists. There are many scientific
problems connected to these explanations and we will highlight some of them alongside
the following alternative history.
Is it possible to make gold from other substances?
|
From a question-answer website:
By adding protons to the atom chemists
can theoretically turning lighter gold atoms with 79 protons
into heavier lead atoms with 82 protons. However, in practice, converting gold
into lead takes a lot of energy and so costs more than the value of the lead
actually made.
The reverse process of converting heavier lead atoms into lighter
gold atoms is also possible. In practice however, tampering with elements at an
atomic level is more likely to produce dangerous unstable atoms of a new
element, rather than sparkling gold.
http://www.sciencenet.org.uk/database/chem/
elemental/c00099d.html
|
Because the element gold is more valuable than for example lead, some people
have tried to make gold from other substances. Although it has been proven that
it is possible to make gold in very small amount in laboratories, we can
conclude that nobody have been rich by creating gold. In common cosmological
textbooks we learn that most of the elements in the periodic table where formed
when big stars formed and later exploded.
From experiments in laboratories we know that we can
make new chemical materials by raising the temperature and the pressure
in an area. Coal and oil may for example be made from organic material in this
way. We have not experienced a massive forming of new atomic elements in laboratories,
but if we have to say something about how the elements in our universe may have
been formed out of the helium gas that filled the first universe, it is probable
that high temperature and high pressure is needed. The only place we probably
find these conditions in our universe is inside stars and if we need more extreme
values, it is always possible to just assume that these elements have been created when stars have
exploded (super nova has been observed from time to time). But there are no
scientific evidence that shows that new elements is forming inside stars or supernovas
today. The main reason for thinking that elements can form inside stars is the
idea that every element have been formed naturally inside our universe after
Big Bang and not
by an external creator. If this assumption is right there just have to be a
transformation process from hydrogen to the elements we know today that
works in our universe. Since we do not observe this process in our universe
today, it must have happened a place we cannot observe it, and that is most
likely inside stars.
Is everything possible in nature, or is there some limits?
Instead of arguing against common cosmological models, we will here just
try to describe some main features with the elements we have in our universe
and also mention where there are scientific difficulties to assume that the different
processes have happened in nature all by them selves. In these cases we think
that it is more honest to mention
a creator than just pretend that those processes may work in the nature although
there are no scientific experiments that support that view. The point here is that there
normally have to be a sort of procedure and experimental equipment behind an
experiment. When these things are absent, the process will not happen. In normal
scientific experiments, man are the creator behind the procedure and the experimental equipment.
We know that man did not create the elements in the universe, but that did not
prove that there where no creator that did these apparently impossible things.
Man can make a car, but nature can not make a care just by itself. It is impossible.
In
general we can say that both God and man is partly outside the nature because
we use something that we can call intelligence to create thing in nature.
The nature would not have managed to make it just by itself. Some people may
disagree on this, and say that everything in nature may have a natural explanation.
This is a faith statement that they may feel comfortable with, but some
people just wants to see some evidence before they accept that sort of statement,
and it is our hope that those people may find some interesting in this text. Those that believe
that Big Bang is a fact and all elements can be formed in nature, are probably not so open minded that they will accept
the arguments in this document and may be they should not bother reading the
rest of the document.
Fundamental
particles
 There
are many elements in our universe and it is not possible to give a full comment
on all features in these elements in a short presentation. Here we will
just give a general presentation based on some basic principle in the EM-model. The EM-model
that is presented here is rather new, and therefore there may be something
in this presentation that need correction. But instead of waiting till everything is checked
in every way, we just present some moments . We will also encourage
those that find something in this text that have to be wrong, to join in this effort
to understand the nature based on an electromagnetic model. Some minor problems in these texts
is not a valid reason
for throwing away the whole EM-model. People have been adjusting the QM-model
for a period of around 100 years to make it fit physical observations, and
it would be wise to spend some time trying to adjust the EM-model to the real
world before concluding that it is wrong.
There
are many elements in our universe and it is not possible to give a full comment
on all features in these elements in a short presentation. Here we will
just give a general presentation based on some basic principle in the EM-model. The EM-model
that is presented here is rather new, and therefore there may be something
in this presentation that need correction. But instead of waiting till everything is checked
in every way, we just present some moments . We will also encourage
those that find something in this text that have to be wrong, to join in this effort
to understand the nature based on an electromagnetic model. Some minor problems in these texts
is not a valid reason
for throwing away the whole EM-model. People have been adjusting the QM-model
for a period of around 100 years to make it fit physical observations, and
it would be wise to spend some time trying to adjust the EM-model to the real
world before concluding that it is wrong.
As mentioned in the first document, there are only
two fundamental particles according to the EM-model, the negative electrons
and the positive protons. But these particles may have different energy and
therefore also different mass. Note here that mass is not a fundamental property according
to the EM-model. More about that in a more complete presentation of how we explain mechanics
according to this model. Here we will just say that Einstein equation: E=mc² says
that mass and energy is two connected properties. The EM-model goes a little further and says that mass or energy is in most cases a matter of more or
less magnetic energy.
Stable and unstable fundamental particles
Before we present the connection between the fundamental particles
according to modern physics / quantum mechanics (Quantum Mechanics=QM) and those we have in the EM-model, we will just
say that concept stable may be interpreted in different ways. In the first universe
we will say that only the hydrogen molecule is stable. That means that those
structures may exist in a natural environment without any spontaneous fision/splitting or
fusion/combination (in) to other structures. This definition may work
on either models.
When the concept stable is used on fundamental particles it normally
means that unstable particles will disappear sooner or later. It is important
to note that it is not logically to use the concept stable connected
to the elementary particles in the EM-model because both the fundamental particles are stable
and there are therefore no need for that concept. Both electrons and protons
are stable in the meaning that they
will never transform into other particles, but at the same time they may have
different energies. On the other hand they may be regarded as unstable
as a single structure because they will spontaneously combine with other particles or structure in
a natural environment.
|
Particles
|
Charge
e=1,60·10-19C
|
Rest mass
u=1,66·10-27kg
|
Lifetime
seconds
|
|
Electron
|
-1
|
0.000549
|
stable
|
|
Proton
|
+1
|
1.007277
|
stable
|
|
Neutron
|
0
|
1.008665
|
1000
|
|
Positron
|
+1
|
0.0000549
|
stable
|
|
Muon
|
-1 or +1
|
0.114
|
2.2·10-6
|
|
Tau
|
-1 or +1
|
1.92
|
2,3·10-12
|
According to the QM-model there are many fundamental
particles and some of them are shown int the table to the left. In the table, there are three particles
said to be stable,
namely: electrons, protons and positrons. The latter is not stable in
a normal environment, so that the meaning of stable her have to be a more theoretical
one that says that they are stable if they are in the
right environment. All the other particle is unstable, meaning that they
will disappear sooner or later. When that happens other
particles are then created. Here we will just say that this "unstable
nature" consisting of fundamental particles that are created and disappears all
the time is not easy to understand. The nature is more predictable according to the EM-model. Therefore we will not
talk about unstable particles excepts when we have to quote from
common literature.
According to the EM-model there are only two fundamental particles. This means
that all particles in the QM-model with positive charge is protons and all particles
with negative charge is electrons. A particle that have a rest mass and no charge
(example: Neutron) then have to be a composition of a proton and an electron.
According to the EM-model the neutron is therefore a structure in the same way as hydrogen
atoms and helium ions are structures. To understand the EM-model it is important
to remember that the neutron is not a fundamental particle as in the QM-model.
A main problem
when comparing the EM-model with the common QM-model of the
fundamental particles is due to the fact that the QM-model have been the accepted
model for a rather long time. The concepts and language is often based on the
QM-model and it is not easy to both absorb a total new model at the same time
as the new model puts other meaning into the words that are used. Before we
get back to the periodic table of the elements, it is necessary to say something
about some of the basic elements in the QM-model.
Neutron is not a fundamental particle - but two
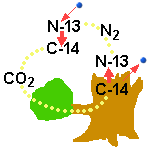
C-14
is a radioactive isotope which can be used to measure how long ago
a tree was cut down. From time to time there will come an electron
from a C-14-atom and at the same time it transforms to a N-13 isotope.
The opposite may happen when an fast electron hit a N-13 isotope.
|
Her we will first argue for the view that neutron is not a basic particle
by mention an example from nature: We know that the isotope C-14 (Carbon) is present in our atmosphere
(as CO2) and that this isotope is unstable with a half-life of 5730
year. That means that there is a possibility that a C-14-atom may be transformed
to a N-13 isotope (Nitrogen) and when doing that it radiate a beta-particle. A
beta-particle is a electron, but because they have rather high velocity, it may be dangerous
and therefore we call
them beta-radiation which is on of three main radioactive radiations. The other
is alpha- and gamma-radiation.
Up in the atmosphere where
there are many electrons with high velocity the opposite process may occur.
An electron may collide with a N-13 and the result may be a C-14 isotope. That
is a fact and not a matter of discussion. But how do the two models
describe these processes. The difference between C-14 and N-13 is that C-14 has
a neutron in the nucleus where the N-13 have a proton according to the QM-model.
That means that a neutron disappear and a proton and an electron are created
when the radioactive C-14 isotope disintegrates. In the opposite process a fast
electron penetrates into the nucleus of a N-13 atom and a proton disappears
and a neutron is created. Is it pedagogical wise to talk
about creation of fundamental particles in such situations which happens all the
time? According to the EM-model it is just a matter of an electron that enters
and leaves a nucleus and that is much easier to understand. According to the
QM-model there are no electrons in the nucleus, and therefore they have to assume
that elementary particles are created and disappears.
Ockham's Razor is the principle proposed by William of Ockham in the
fourteenth century: ``entities should not be multiplied unnecessarily''.
In short we can say that it the simplest explanation or model is the best. It
is in connection where we have more than one competing theory which describe the same phenomena, that
this principle is useful. |
According to Ockham's razor, it is not a good thing to make a description
more complicated than it have to be, unless you just want to create confusion
to avoid questions that may reveal weaknesses in a theory. Here we will just
conclude that a neutron may be considered being an electron and a proton. Later
we will mention what makes a neutron different form a hydrogen atom
that also consist of a proton and a electron. But here we will just conclude
that it is primarily a matter of useful concepts and not a matter of scientific evidence,
when we here discuss if a neutron is created from a stable electron and proton
when a N-13 transform to a C-14.
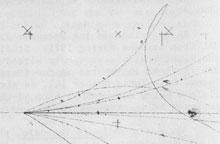
Positron - a proton with no spinn?
Before we comments on positrons which is a sort of exotic particle that don't
exist in nature, it is important to know the principle of how scientists have discovered
the many atomic particles that we find in different tables. Those in the table
above is only some of them. The picture on the right shows tracks of fragments
from a collision in a chamber with a homogeneous magnetic or electric field.
Positive charged fragments will then be deflected in one direction and negative
charged fragments in the other direction. Velocity and mass will then determine
the slope of the curve, and from the curves it is then possible to calculate
back and find the charge and mass of the fragments.
In these sort of experiments there have been observation of positive
charges with a mass like an electron, and they have got the name positron. According
to the EM-model, it is the spin of the particle that determine the mass, and
it is therefore no problem to assume that a proton may lose all its spin and get
a mass like an free electron.
The problem for that positron is that it will be attracted to a near by electron and
because none of them have an initial spin which can cause magnetic repel,
they will rapidly disappear. Here we will just conclude
that we do not find positrons in nature and those that have been produced in
particle-accelerators are not stable in our universe because of the many electrons
it can meet. A table that says that positrons is stable is therefore based on a
theoretical basic and not practical observations.
Muon and Tau - just protons and electrons with other spinn?
In the table above we have included Muon and Tau as example of charged particles
that may be positive and negative, but have different masses from ordinary protons
and electrons. As mentioned above, protons and electrons may have every value
of spin seen from a theoretical point of view. The fact that we don't find particles with
all masses (all spins) in nature, may at first look like an argument against the EM-model. But according
to the EM-model it is not expected that the particles will get all values of spin.
It is not a easy task to show that, and here we will just make some short statements
connected to this problem. A general point is that we are used to think
mechanically. The QM-model is one example how one have tried to understand
nature based on mechanical concepts. Note that the basic for modern physics
is called quantum mechanics. The EM-model on the other hand is not based
on a mechanical fundament, but a electromagnetic fundament, and in this "new
world" concepts as mass, friction and other do not
exist. We us them in normal life, an also in this document, because they is an important part of our language, but if we shall explain what is behind
those concepts, we have to use other words to make a consistent theory.
Some basic assumption in the EM-model:
Energy a common concept in both a electromagnetic and a mechanical world
view and here follows some basic assumptions connected to that concept.
1 There are two sorts of energy, a static one that is connected to the electric
field (potensial energy) and a dynamical one that is connected to the magnetic
fields which is relative movement of electric field (kinetic energy).
Here we will call the first electric energy and the second magnetic
energy in stead of potential energy and kinetic energy as common in a mechanical
world. The magnetic energy can also be divided into two different forms,
one connected to spinning charges (that turns around its own axis) and one connected
to charges that moves in space (in one direction). The first may be called spin
energy and the second may be called velocity energy.
2 We will first assume that there are no friction connected to moving electric
fields and that means conservation of energy in all movements
of charges in the universe. Protons that was initialised with spin at the time of creation, will then
keep spinning forever if noting happens.
When the electrons then started to move toward a proton we can talk about a
loss of electric energy that turns into velocity energy and the
result when the electron stops near the proton is spin energy. We then
assume that the total energy is conserved in this sort of processes and therefore
we would expect that elements with the same number of particles would have the
same amount of energy connected to them.
3 Observations shows that when electrons falls into atoms we will experience
an electromagnetic radiation out from the structure. That means a loss of energy
from the structure and here we will just mention that the EM-model is not dependent
on a total conservation of energy as standard mechanical models seems to. In general
we can then say that the energy connected to the nucleus of the elements is
huge, compared to the energy connected to oscillating electron and binding electrons
in chemical compounds. An example: If we burn wood (carbohydrates) there is
a chemical reaction which release heat energy. According to the EM-model and
also modern physics (E=mc²) there should be a loss of mass in the sum of components that is involved from before to after the process.
The mass will decrease when energy go out as electromagnetic waves. In practical situations it is only possible to
measure that sort of weight loss in atomic processes and not in chemical processes.
The reason is a huge gap between chemical and atomic energies. It is possible
and also probable, according to
the EM-model, that there may be some small energy losses every time we get
radiation from components that have captured an electron.
According to QM-model
energy in radiation are quantified and all the energy is restored when the photon
is absorbed by another component. This means that people who believe in the
QM-model have problem in accepting that there is a loss of energy when
we for example experience negative interference between two beams of light.
Those that believe in the EM-model seem to be more open minded. As long
as nobody have given a logical explanation of how energy may be conserved in
for example laser experiments where we experience destructive interference,
they accepts that there may be a small loss of energy in our universe.
But if there is a sort of "running down mechanism" in the universe,
it have to be very slow and therefore hard to measure directly.
Why do we not find particles with all possible spin values?
| 

According
to the em-model a Muon is a electron (or proton) with a size between
a free electron and a proton. The reason is the spinn energy
that have a level between the normal spinn of a electron and a proton.
|
The QM-model is based on common observations that show that we often find discrete energy
levels in nature. The way the QM-model explains that is just to assume that
separate energy levels are the fundamental property in nature. Therefore
it is not possible to explain this property based on a more fundamental principle.
It may seem a little unfair that those who believe in the QM-model don't have
to explain the discrete energy levels, because it is the basic assumption, but
other have to. According to the EM-model the main reason for observing just
some discrete particle energies (masses) like Muons (electrons and protons according
to the EM-model) coming out of an collision is of cause that all particles in
the structures have special positions where they are stable. In general we experience
that most of the particle in a structure is so stable that they do not come
out, but some have a special exposed position and here we assume that it is
those that comes out as for example Muons after a collision. In the table above we
sees that a Muon have a mass which is about 1/10 of a proton and 200 times the
mass of an electron. That means that an electron (or proton) have still much
spin left when it have left the structure where it came from. But it is probably
not the same spin as it had in the structure because some of the initial spin
energy must have been transformed to velocity energy and electrical
energy. Experiences show that Muons are not stable and according to the
EM-model it will sooner or later meet some other structure and transform to for
example a free electron that means it looses more of its spinn.
Another problem is to explain why structures seems to end up with the same
energy levels after some recombinations. In general we will here assume that
if there are a loss of energy in a recombination on atomic level, it have to
be extreme small compare to the energy that are involved and it is therefore
difficult to measure the energy loss. Above we have mentioned negative interference
as a mechanism for energy loss, and if that is the only way energy may disappear,
then we can say that practically all energy involved in a recombination have
to end up in another energy form. Over time the particles that receives
or loss energy will adjust themselves to each other and end up in the normal
stable energy levels.
What is the difference between a neutron and a hydrogen atom?
| 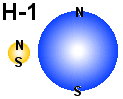

The
figure shows a hydrogen atom (H-1) and a neutron(n) as they
may look lik according to the em model.
|
In the figure to the left we se both a hydrogen atom (H-1) and a neutron
(n) as they may look like according to the EM-model. Note that the scale is wrong
and the proportion between the distance and the size of the particles is much
higher in nature. In both cases we have a structure composed of a proton and
an electron. It is then electric force that keeps them together while it is
magnetic forces that keeps them from falling into each other. According to the
EM-model the size of the particles in a hydrogen atom has to be different, but
the background for the sizes in the figure is graphical considerations (how
to draw clear structures) and not scientific considerations. Note
also
that we in the figures have placed letters that mark where there is a magnetic
north pole (N) and a magnetic south pole(S). The reason for that is to make
it easier to see where there are magnetic repel or attraction. In both cases here
we have a repel because same poles are in the same end of the particles.
A main question that is not settled yet is the size (or mass) of the particles
that forms a neutron. Here we have given them the same size, because it makes
it easy to recognise neutrons in drawings. Some more studies have to be done
to settle the size of the particles in neutrons, but the conclusion so long
is that they have to be between the size of a normal proton and a free electron.
A scientific argument for the particles in a proton being the same size is connected
to Muons that seems to have same mass both when they are positive and negative.
A source for electrons an protons with same mass coming out of atomic structures
may be a smaller structure where those two particles have the same size. It
is just a sort of guess, but also that sort of guesses may lead to some useful
information.
|
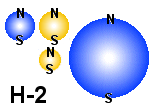
The
figure is a model of a Deuterium atom which is present in "heavy
water".
|
The first to note about neutrons is that they are only stable in other structures
and not alone. That means for example that a neutron is stable inside a Hydrogen
atom (or Hydrogen molecule). That structure is called Deuterium and a possible
structure of a Deuterium atom is shown to the right. Note that the poles may
be titled different from what shown in the figure, but here too is it more easy to see what poles that is attracting each other and what poles that repels each other
if all magnets have their axis in the same direction. As mention earlier, Hydrogen
atoms are not stable and here we assume it also applies to Deuterium. Therefore
two atoms will stick together and make a molecule. But in stead of trying to
draw all possible structures we will her just pick up some structure that shows
special important principle and leave the other to somebody that have a deeper
background in the subject.
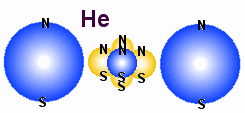
Helium and alpha particles - what do they look lik?
| 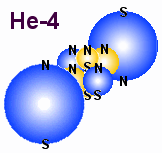
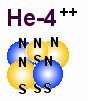
A
figure that shows a possible structure for helium and its nucleus.
|
The next structure that should be mentioned in this introduction to atomic
structures is Helium which normally is a gas and its nucleus which is often
called alpha-particle. The latter seem to be more stable structure than many
other atomic structures because it keeps its structure in most recombinations
and comes out as stable structure when
other bigger unstable elements disintegrates.
In general we can say that a helium atom is stable because it has two outer
electrons rather close to each other. They therefore do not acts as unpaired
electrons that attracts other unpaired electrons. Therefore Helium do not form
molecules, but a single atom behave as a gas and therefore the outer form must be similar
to H2 gas which also have an electron in each end. The fact that Helium
is a gas and the nucleus must be between the two outer electrons indicates that
the nucleus is rather small compared to the electrons. A more complete argument follows
later where we will discuss the basic conditions for being a gas.
A main problem here is to figure out the structure of the helium nucleus which is
build of 6 particles, 4 protons and 2 electrons. The figure above shows one
possible structure where all magnetic spins have the same direction. It seems
to be the easiest way to draw a structure that may be stable because electric
and magnetic forces may balance each other, but it is not easy to tell if this
is the most stable structure. That is not important either. The main point her
is that a structure of those 6 particles is stable exist in the nature, and here
I just will encourage others to search for a better structure which can explain
all known features of Helium and the alpha particle.
What happens when more particles are put into the nucleus?
The periodic table of the elements shows that the general rule when
moving from an element to the next, is two protons and 1 electrons more in the
nucleus. (1 proton and on neutron according to the QM-model.) Note that
we here define the nucleus to be all the atom except the outer most electrons
that is involved in chemical bindings. In general there may exist other stable
isotopes, but here we will just comment on this general rule by using the step
from Helium (He-4) to Litium (Li-6) as an example. Helium(He) has atom
number 2 and Litium (Li) has atom number 3.
Forming new elements from hydrogen is not a natural process
In
general it is not easy and usually not possible in a laboratory to build
new elements by putting new particles into the nucleus. It is definitive not
a process that we can see goes on in the nature today and therefore we here assume
that there have to be a creator involved in making bigger elements out of smaller
elements. Some books say that this sort of process goes on inside stars today,
but as long as we don't se any description of natural mechanisms that can do
the job, it is most likely that this is wishful thinking and not real science.
Here we then assume that the creator is the only one that can put new particles into the
nucleus with the right energy. When the nature then " takes
over", the nucleus will recombine and make a stable structure. Here follows
some comment on some general principle connected to how the nucleus would
behave according to the EM-model.
Spin from outer electrons dominate outside the atom
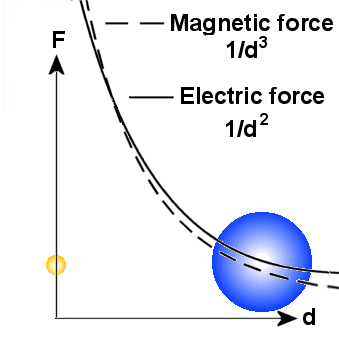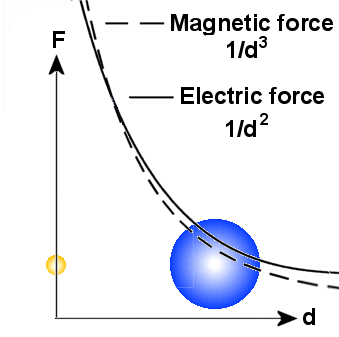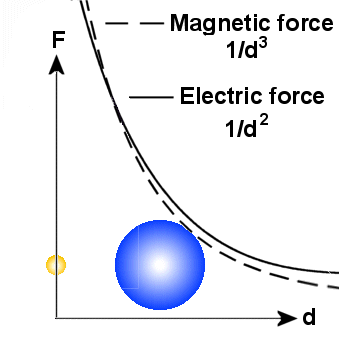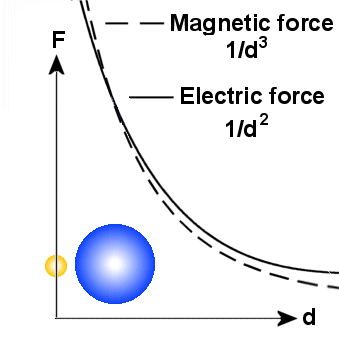 A main principle
is that the there are more energy involved in small particles than bigger on.
When a small particle transform energy to a bigger one, it became bigger,
but the other particle gets smaller. Because the electric field varies as 1/d²
around all particles, it will in general be stronger than the magnetic field
in a distance. Note also that the variation in diameter of the particles do
not affect the electric field.
A main principle
is that the there are more energy involved in small particles than bigger on.
When a small particle transform energy to a bigger one, it became bigger,
but the other particle gets smaller. Because the electric field varies as 1/d²
around all particles, it will in general be stronger than the magnetic field
in a distance. Note also that the variation in diameter of the particles do
not affect the electric field.
The magnetic field which varies as 1/d³ will
then dominate near to the particles. But it is also important to note that the
magnetic field will be affected by the diameter of the particle and a general
rule is that the magnetic field from a big particle reach longer out than that
from a small particle if the spin is the same. According to the EM-model, the
spin increases when the particle gets smaller, but in sum we will here assume
that spin from big particles reaches longer than spin from small particles.
In praxis, this means that spin from outer electrons, and not protons or those
electrons that are captured in the nucleus, will dominate in the area
around the
atom.
The nucleus - a positive ball shaped structure
Because the structure
of the nucleus is in three dimensions, it is not easy to figure out how it look
like on a two dimensional paper/screen, and therefore we will just assume that there
is a stable structure and we will draw it as a ball shaped formation consisting
of protons and electrons with mostly the same size. It is probable that
there will be a variation in size, with the smallest particles in the middle,
but it is not so easy to show this in a drawing. In general we also draw protons
and electrons with the same size and one such couple may then be regarded as
a neutron. In general there have to be some negative particle to make some electrical
attraction and arrange the strong magnets in different directions so that the
whole structure become stable although it is dominated by positive protons that
naturally will repel each other.
|
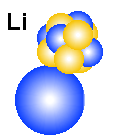
A
litium atom (Li-6) as it may look like according to the EM-model.
|
From Helium (He-4) to Litium (Li-6)
As mention above, the nucleus of helium is small and stable, and forcing
new particles into it would mean that the whole structure have to be rearranged
probably to a bigger and less stable structure. In general it may be that it
would be easier to force two helium atoms together and first make a carbon atom
(C) and then pick out some particles from the nucleus to form litium (Li), but
the main point here is not how the creator did it, but what will happen
naturally when for example 6 protons and 3 electrons are forced together
into a nucleus. If the creator for example started with a helium atom
and forced 2 protons and 1 electron into its nucleus, we would
expect a new structure that will start to interfere with the surroundings because
it would be less electric and magnetic neutralized seen from outside. A possible
next step is that the two outer electrons from the helium gas will be sucked
into the nucleus to form a more stable structure. The whole structure will then
have one more positive charge than negative charge and therefore it will attract
a new electron. At that time the "holes" in the nucleus will
be filled, meaning that the nucleus have become a rather stable structure. That
means that the last electron will end up as an unpaired electron with a relative
weak connection to the nucleus (electric attraction). Note that Li-7 and not
Li-6 is the most common isotope, but the principle mentioned her will work for
all elements.
Periodic properties according to the em-model
The periodic properties in the elements need an explanation and her follows
a short description of some main features. A main reason for the periodicity
of the elements is that it is possible to place at most 4 pair of electrons in
a layer around a nucleus before the next electron that is added is forced further
out in a new layer. It is expected that it may be shown mathematically based on a electromagnetic
model, but we are not able to present it her. In stead we will just mention
some basic principle according to the EM-model and just show three different
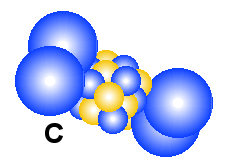
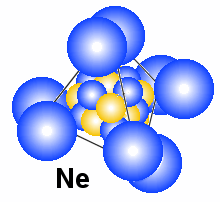
drawing of how the elements may look like. The three elements are Litium, Carbon
and Argon as shown in the table below.
Note that there are different isotope in most of the elements and therefore
we can conclude that it is the outer electrons and not the nucleus that is the
main reason for the different chemical properties. These properties is therefore
determined by the number of electrons left outside of the nucleus after the
outer part of the nucleus have been closed with a "neutralized coating".
This means that the charges outside the nucleus will feel a spherical
symmetrical electric field due to the dominance of positive charges in the nucleus
and relative small magnetic fields from the nucleus. It is of course properties
inside the nucleus that determine the charge and as a secondary effect, the
other fundamental properties
with the elements. But here we will concentrate on the electrons around the
nucleus and show how the EM-model can explain different chemical an physical
properties whit the elements.
Litium is the first element in the row 2 in the periodic table.
We have commented on that element before and here we will just mention that
the lonely electron will have a spin that acts as a rather strong magnet which
will attract another electron with same properties. We should therefore expect
that Litium would form molecules (pairs of atoms), but because Litium is not
a gas, it is not common to speak of Litium molecules.
When we go from Litium to Carbon, it means adding more electrons
outside the nucleus parallel to a corresponding higher value of the positive
charge in a slightly bigger nucleus. Carbon have four electrons outside
the nucleus according to the EM-model. Because all of them have a negative charge
somebody may expect that they would be placed as long as possible from
each other in a tetrahedral form. That is not the case and the reason is the spin that makes each
of them a magnet. Therefore they will stick together two and two and form an
atom which may look something like the drawing above.
If we continue to add electrons around the nucleus and charge in the nucleus
we ends up with Neon that also have a drawing above. Lines are added to make
it clearer that the electron pairs make a tetrahedral form. The main roles that
determines the electron structure is that magnetic properties makes them
go together two and two if possible and the electric properties that tries to
place the electron pairs as long as possible fro each other.
If we tries to add one more electron the structure, the electron pairs
will not let another electron enter into this structure. That means that the eight electrons will be
a part of a structure that interfere little with outer electrons and therefore
they can be included in what we here calls the nucleus. The extra electron will
start to make a new outer structure with one electron outside the nucleus. This
element have the symbol Na and it have many feature common with Litium.
The octet rule
In chemistry we have an octet rule which says that Atoms proceeds
as far as possible toward achieving an octet by sharing or transferring electrons.
The main reason for this rule is the same principles as we have described above.
Unpaired electron attracts other unpaired electrons because of magnetic forces
and it may at most be 4 pair of electrons around the different nucleus in a
chemical compound.
Gas will expand while liquid and solids stick together - why?
As shown above, the chemical aspects with the elements may be explained with
help of the EM-model. There are also some physical aspects connected to the
different elements
that need an explanation. A main question her is why some elements
forms gas and other elements forms liquids or solids at normal atmospheric
pressure. Although this question seems to be a fundamental question, it seems
to be ignored in common literature and the reason may be that the QM-model
and the kinetic theory of gases can not explain why Oxygen(O2) and Carbon
dioxide(CO2) forms gasses, but Carbon do not.
The most obvious difference between gas and liquid/solids is the fact that
gas will expand if there are free space around, but the others will stick together.
A natural conclusion is that there are a repelling force between gas molecules,
and a attracting force between liquid/solids. According to the kinetic theory of
gases, there is no repelling force, but the reason for what we call gas
pressure is high velocity of the gas molecules. We will not discuss the different
problems that follows from that assumption, but instead show how gas pressure
is explained according to the EM-model.
|
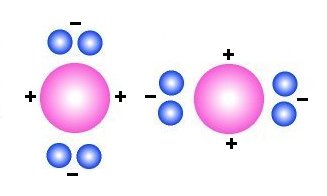
Two
Carbon atoms will have different charge in different ends and therefore
opposite charges will attract each other and Carbon therefore form
solids (graphite or diamond).
|
At first we chose carbon as an example of those elements that
don't form gas. In stead of drawing the nucleus as many particles, we here then
just draw it as a sphere with red colour that means that it have a positive
charge in contrast to the blue electrons that have a negative charge. The figure
to the left then shows two carbon atoms. We have also marked with signs some
positive and negative ends of the structure, and we would therefore expect that
opposite charges will attract each other. The octet rule will then "try"
to get four pairs of electrons around each nucleus, but in this case it is easier
to form graphite than diamond and therefore most carbon have three pair of electrons
(not four) around the nucleus.
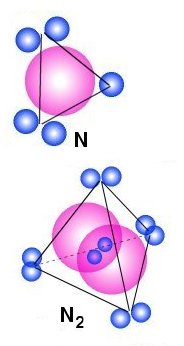 Nitrogen
is a gas
Nitrogen
is a gas
A nitrogen atom has one more electron that a Carbon atom and
to the right we see how the electrons may be placed around the nucleus. Because
the two pair of electrons and the unpaired electron all have negative charge
they will repel each other. But because the unpaired electron is a rather strong
magnet, it will attract another electron with same properties, and because of
that, Nitrogen will form molecules as shown in the figure below. One pair of
electron will then be placed between the two nucleus and the other will then
be placed as long as possible from each other. That means that they will form
a tetrahedral form as the lines indicates. The main question is the where are
the positive and negative ends of this structure.
Mathematical calculation shows that this structure have only negative ends! If we calculate the sum of electric force
from the positive and negative groups of particles, onto a negative electron in
a distance from the structure, we will always get a repel (see example
below). That means that this structure
will only have negative ends or corners and it will always repel other similar
structures. There are two exceptions: If the free electron gets to close there
may be positions where the electrical attraction will dominate over the repel.
In praxis that means that by high pressure it is possible to force a gas to
transform to a liquid or solid. If we on the other hand place the electron
in infinity, the sum of attraction and repelling force will be zero.
In our simple mathematical models that we have used so long we have assumed
that both nucleus and the two binding electrons is a single point charges and
the same are the other electron pairs. Somebody may say that this is an over simplification
that may give wrong results. Our response is that all mathematical models is based
on simplifications of the real world, and the results from calculations
will never be exact. A reason for using simple mathematical models is that they
are easy to perform and check . The main reason for collecting many charges
in few points is the fact that the nucleus according to all atom models is much
smaller than the mean distance between the particles (a factor of 104)
and if we assume that the electron pairs is placed halfway between the nucleus,
we clearly see that the figures here have wrong proportions. That mean
that if the electrons and the nucleus in the figure her should be drawn with
right size compared to the distance between them, they should be like points.
How to calculate the force between a electron and a tetrahedral
structure?
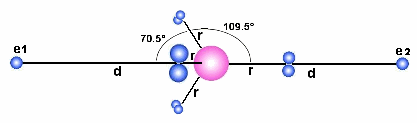 The
figure to the right shows a tetrahedral
structure with a free electron to the left (e1) and to the right (e2). The Form/JavaScript
below calculate the force between the tetrahedral structure and one of the electrons.
The distances d (between the center of the tetrahedral structure and
the electrons) and r (the radius from the center of the tetrahedral to
the electron pairs) is measured in pm (pico-meter =10-12m)
and different values may be put into the fields to the left. By pushing one
of the buttons in the middle, the Form/JavaScript calculates the force between one
electron and the tetrahedral structure. A main point her is to observe that
the force become repulsive for most combinations of values and that means that
elements and molecules with that sort of structure is a gas under normal conditions.
The
figure to the right shows a tetrahedral
structure with a free electron to the left (e1) and to the right (e2). The Form/JavaScript
below calculate the force between the tetrahedral structure and one of the electrons.
The distances d (between the center of the tetrahedral structure and
the electrons) and r (the radius from the center of the tetrahedral to
the electron pairs) is measured in pm (pico-meter =10-12m)
and different values may be put into the fields to the left. By pushing one
of the buttons in the middle, the Form/JavaScript calculates the force between one
electron and the tetrahedral structure. A main point her is to observe that
the force become repulsive for most combinations of values and that means that
elements and molecules with that sort of structure is a gas under normal conditions.
Comment to the calculation:
By using Coulombs law:
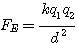
it is rather simple to calculate the electric force (FE) between
a molecule/atom with a tetrahedral form and a free electron (e1 or e2). In
the equation d means the distance between two charges. Since a tetrahedral
structure have 5 clusters of charge we have to calculate their individual distance
from the free electron and sum up the forces to get the total force. In the
figure d means the distance between the electron and the center of the
tetrahedral structure, while r means the radius from the center to each
of the electron pairs around the nucleus. The equation gives the force in N
(Newton) if we use the denomination C (Coulomb) for the charge. The constant
k=8,9875·109N·m²/C²
and e=1.6021·10-19C (the
elementary charge) . Because three of the electron pairs
is symmetrical positioned around the axis on the figure, we will find that their
components normal to the axis will vanish and therefore we only calculate the
parallel component to the axis which is the same for all and therefore
we just multiply with 3 (and get 6 electrons). The total equation is for
e1 is:
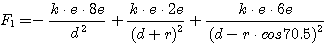
The equation for F2 is similar with some signs changed.
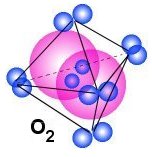
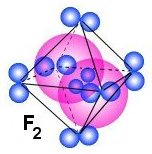
Different structures that make elements to form gas
If we continue from Nitrogen and upward in the periodic table we get Oxygen,
Fluorine and Neon which all is gas under normal conditions. The figure above shows
how these molecules may look like and we see that the main structure may have
4, 5 or 6 pairs of electrons around the central part of the gas structure.
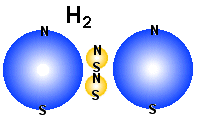
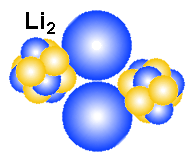
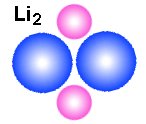
Why are hydrogen and helium gas?
Why are hydrogen and helium gas, while Litium that have the same number
of electrons, are not?
In the figures above we show how the first three elements in the periodic
table may look like, both in the blue-yellow particle model and the blue-red
electron-nucleus model. It is obvious that Litium are not a gas either as atoms
or as molecules. The reason is positive and negative ends in the structure. A main point
in all the drawings of hydrogen and helium is that the nucleus is smaller than
the electrons. That means that the positive nucleus may experience a sort of
hiding behind the negative field from the electrons. Note that the electric
force that varies as1/d² tells that it is what is
nearest that will result in the strongest electric field. From every side
of the structure a free electron will experience that negative charge is
most close and therefore the repel from these charge will dominate over the
attraction from positive parts that are a little longer away. This means that
the size of the nucleus in the drawing of carbon to neon earlier in this document,
probably is to big compared to the size of the electrons. The drawings are made
in different periods of the study of the EM-model and in stead of adjusting
the figures now, it is better to wait until a complete model with values for
the different sizes is developed. It is my hope that somebody that reads this
may want to go into a mathematically model of the elements according to
this EM-model and from that work find some real values for size and spin of
the particles.
The second day
|
1:6 |
And God said, Let there be a firmament in the midst of the waters, and let
it divide the waters from the waters. |
|
1:7 |
And God made the firmament, and divided the waters which were under the
firmament from the waters which were above the firmament: and it was so. |
A main idea behind this presentation of the EM-model is to introduce basic
elements in the universe before the more complicated elements that explains
life on earth. The first document was about the fundamental particles in the
universe and this chapter is about how the elements may have been formed.
A main inspiration for this work have been the creation story in the Bible and
here we will just sum up this document with a comment of what happened the second
day of the creation according to the Bible. See the frame to the right. If we
assume that the universe started with mainly hydrogen gas, as mentioned in the
first document, we face two main problems when we shall try to explain how the
elements have been formed.
1 Hydrogen is a gas which will expand and not concentrate in to stars.
The gravitational force, that common literature says have resulted in a clumping
together into stars, are far to weak to overcome the gas pressure which may
regarded as a force with opposite direction. A conclusion is therefore that
we know of no force in nature that may form the first stars out of hydrogen
and therefore an honest scientist should admit that help from a creator seems
necessary.
2 To form new and bigger elements from smaller elements when Hydrogen
is the starting point is not a normal process that we find in nature today.
Experiments in laboratories shows that very high values of energy in very small
area are needed to make new elements in the lower part of the table. But it
is not enough with much energy. Too much energy normally ruins a process where
the goal is to build something that are not a natural product in a common process.
Man have some experiences in building chemical structures. A main problem in
these processes is to position the right elements at the right place to the
right time and that sort of processes normally needs an intelligent person which
is able to make necessary equipment and perform the process. It may therefore
seem a little arrogant when we hear that people that have no experience with making
the common elements, claiming that those processes may happen in the center
of big stars without any sort of help from a creator. Our conclusion here is
that the most important learning from scientific experiments is that something
in nature do not happens by itself if we just supply enough energy. We do not
expect a car coming out of an explosion in a car factory. Therefore there have
to be a creator behind the forming of the elements.
It is also interesting to see that processes connected to these main problems
which are mentioned her, is also mentioned in the Bible when the second day
is described. At first we will just say that word "water", probably
is used as a general word for the first homogenous matter (Hydrogen) as mentioned
in the first document. The main message from the Bible text seems to be that the
creator interfered with the result from the first creation day on the following
day. "Divide" is her a main concept and it may mean that he divided
the matter into different elements so that some sank down (solids and liquid)
and some formed a firmament which we here interpret as the atmosphere which
then where filled with gasses. A possible interpretation of the water above
the firmament is that the creator did not do anything there at that second day. It is
recorded that he formed the sun and moon on the forth day and it is therefore
probable that he formed the outer universe later. That may for example explain
why the earth is not so hot as most of the big heavenly bodies are today. The
earth have got more time (and external help) to cool down.
The main conclusion here is then that the problem to collect matter to a
ball shaped structure (our earth) with enough mass to make a gravitational field had to
be done by a external creator. Also the division of mater into different elements cannot
happen without help from outside nature. Is it a coincidence that the editor
of the Bible seems to be aware of these two main problems, but most science
literature seems to ignore them?
 In
the previous document we have presented the two fundamental particles (electrons
and protons) and the two fundamental
forces in nature (electric and magnetic force). We also saw that a universe filled
with hydrogen gas is a probably first step toward the universe we know today.
In
the previous document we have presented the two fundamental particles (electrons
and protons) and the two fundamental
forces in nature (electric and magnetic force). We also saw that a universe filled
with hydrogen gas is a probably first step toward the universe we know today.
 There
are many elements in our universe and it is not possible to give a full comment
on all features in these elements in a short presentation. Here we will
just give a general presentation based on some basic principle in the EM-model. The EM-model
that is presented here is rather new, and therefore there may be something
in this presentation that need correction. But instead of waiting till everything is checked
in every way, we just present some moments . We will also encourage
those that find something in this text that have to be wrong, to join in this effort
to understand the nature based on an electromagnetic model. Some minor problems in these texts
is not a valid reason
for throwing away the whole EM-model. People have been adjusting the QM-model
for a period of around 100 years to make it fit physical observations, and
it would be wise to spend some time trying to adjust the EM-model to the real
world before concluding that it is wrong.
There
are many elements in our universe and it is not possible to give a full comment
on all features in these elements in a short presentation. Here we will
just give a general presentation based on some basic principle in the EM-model. The EM-model
that is presented here is rather new, and therefore there may be something
in this presentation that need correction. But instead of waiting till everything is checked
in every way, we just present some moments . We will also encourage
those that find something in this text that have to be wrong, to join in this effort
to understand the nature based on an electromagnetic model. Some minor problems in these texts
is not a valid reason
for throwing away the whole EM-model. People have been adjusting the QM-model
for a period of around 100 years to make it fit physical observations, and
it would be wise to spend some time trying to adjust the EM-model to the real
world before concluding that it is wrong.






 A main principle
is that the there are more energy involved in small particles than bigger on.
When a small particle transform energy to a bigger one, it became bigger,
but the other particle gets smaller. Because the electric field varies as 1/d²
around all particles, it will in general be stronger than the magnetic field
in a distance. Note also that the variation in diameter of the particles do
not affect the electric field.
A main principle
is that the there are more energy involved in small particles than bigger on.
When a small particle transform energy to a bigger one, it became bigger,
but the other particle gets smaller. Because the electric field varies as 1/d²
around all particles, it will in general be stronger than the magnetic field
in a distance. Note also that the variation in diameter of the particles do
not affect the electric field. 

 Nitrogen
is a gas
Nitrogen
is a gas The
figure to the right shows a tetrahedral
structure with a free electron to the left (e1) and to the right (e2). The Form/JavaScript
below calculate the force between the tetrahedral structure and one of the electrons.
The distances d (between the center of the tetrahedral structure and
the electrons) and r (the radius from the center of the tetrahedral to
the electron pairs) is measured in pm (pico-meter =10-12m)
and different values may be put into the fields to the left. By pushing one
of the buttons in the middle, the Form/JavaScript calculates the force between one
electron and the tetrahedral structure. A main point her is to observe that
the force become repulsive for most combinations of values and that means that
elements and molecules with that sort of structure is a gas under normal conditions.
The
figure to the right shows a tetrahedral
structure with a free electron to the left (e1) and to the right (e2). The Form/JavaScript
below calculate the force between the tetrahedral structure and one of the electrons.
The distances d (between the center of the tetrahedral structure and
the electrons) and r (the radius from the center of the tetrahedral to
the electron pairs) is measured in pm (pico-meter =10-12m)
and different values may be put into the fields to the left. By pushing one
of the buttons in the middle, the Form/JavaScript calculates the force between one
electron and the tetrahedral structure. A main point her is to observe that
the force become repulsive for most combinations of values and that means that
elements and molecules with that sort of structure is a gas under normal conditions.